What Is The Expected Size Of The Plasmid Plus The Cut Foreign Dna
Congratulations, y'all have a plasmid expressing your gene of interest (YGOI) and are ready to swoop into your functional experiments! Whether you've cloned the plasmid yourself or obtained it from a colleague downward the hall, information technology is always a proficient idea to take some fourth dimension to confirm that you are working with the right construct, and verify that the plasmid you received matches the expected sequence. Here at Addgene, we use NGS-based quality command to confirm the sequence of all the plasmids we distribute. This method is fourth dimension-intensive, then we recommend a diverseness of ways to screen and verify your plasmids. Here, we'll cover restriction digest assay. Diagnostic digests tin can be used to confirm the rough structure of the plasmid based on the predicted sizes and organization of dissimilar featureswithin the plasmid. Restriction analysis tin can also exist used successfully fifty-fifty if you don't have the total plasmid sequence. Once you have purified plasmid DNA, this method can be done right in your lab in less than a day. Diagnostic brake digests are comprised of 2 split steps: 1) incubating your DNA with restriction enzymes which carve the Dna molecules at specific sites and two) running the reaction on an agarose gel to determine the relative sizes of the resulting Dna fragments. Brake digests are ordinarily used to confirm the presence of an insert in a particular vector by excising the insert from the backbone. To do this, you'll use enzymes with restriction sites that flank the insert. You will demand to know both the approximate size of the vector backbone besides every bit the predicted size of the insert. You tin search NCBI for YGOI to find the particular reference sequence if necessary. Scout this video for a quick overview of how to analyze a restriction digest: The following tips will make it easier for you to obtain a useful and informative diagnostic restriction digest. More Plasmid Eductional Resources: 
Diagnostic restriction digest
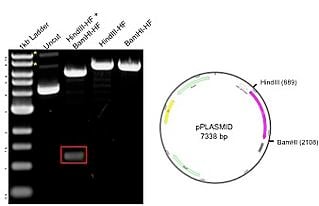 The example plasmid on the right has a total size of 7.3kb, including a ane.two kb insert. The plasmid was digested with 2 unique enzymes (HindIII and BamHI) and run on an agarose gel. The resulting gel paradigm includes a 1kb ladder (lane 1) that has bands ranging from about 500bp to 10kb, with the iii.0kb fragment having increased intensity to serve equally a reference band. The uncut DNA (lane 2) shows three possible plasmid conformations, with relaxed and nicked marked with asterisks (*). When the plasmid is digested with eitherHindIII and BamHI lone (lanes four-v), at that place is a single ring of 7.3 kb representing the full size of the plasmid. The double digest with both HindIII and BamHI (lane 3) produces bands at 6kb and 1.2kb (blood-red box), matching the backbone and insert, respectively. The results on the gel correspond to the predicted sizes.
The example plasmid on the right has a total size of 7.3kb, including a ane.two kb insert. The plasmid was digested with 2 unique enzymes (HindIII and BamHI) and run on an agarose gel. The resulting gel paradigm includes a 1kb ladder (lane 1) that has bands ranging from about 500bp to 10kb, with the iii.0kb fragment having increased intensity to serve equally a reference band. The uncut DNA (lane 2) shows three possible plasmid conformations, with relaxed and nicked marked with asterisks (*). When the plasmid is digested with eitherHindIII and BamHI lone (lanes four-v), at that place is a single ring of 7.3 kb representing the full size of the plasmid. The double digest with both HindIII and BamHI (lane 3) produces bands at 6kb and 1.2kb (blood-red box), matching the backbone and insert, respectively. The results on the gel correspond to the predicted sizes.Restriction digest tips and tricks:
For your digest:
For your gel:

What Is The Expected Size Of The Plasmid Plus The Cut Foreign Dna,
Source: https://blog.addgene.org/plasmids-101-how-to-verify-your-plasmid
Posted by: zimmermanwidat1975.blogspot.com


0 Response to "What Is The Expected Size Of The Plasmid Plus The Cut Foreign Dna"
Post a Comment